
Advanced continuous manufacturing (CM) is emerging as a preferred platform to produce full finished injectable liquid dosage forms. In this work, a novel plant and digital twin model of continuous manufacturing of full finish final liquid dosage forms has been developed. The developed continuous injectable manufacturing process takes the API synthesized and purified via a continuous API manufacturing process, either in powder form or as a solution in the final liquid ingredient of the formulation and turns it into a finished liquid product. This process is composed of three flexible modules that can be easily re-configured and adapted to manufacture a range of drugs involving liquid dosages forms. The developed digital twin model library consists of the mathematical model of unit operations involved in modules 1-3.

Continuous manufacturing methods have been extensively demonstrated to have a significant impact on improving quality, reducing manufacturing costs, and increasing productivity across various industries, ranging from automobiles to fine chemicals to food and consumer products. Within the pharmaceutical industry, these and other benefits have provided impetus for rapidly growing implementation of continuous manufacturing off solid dose products.
This work aims to extend the utilization of advanced manufacturing methods to the second-largest category of pharmaceutical products: liquid formulations, which include oral liquids, aerosols, and liquid injectable. Similarly, for liquid products, we anticipate that the implementation of a small-scale fully capable system that minimizes complexity while maximizing flexibility will enable many companies to pursue implementation.
The proposed system, adapted for purpose, can be an essential component of a strategy to strengthen the US supply chain and to address perennial shortages of injectable drugs. Similarly, flexibility in the compounding/sterilization/fill finish module will enable manufacturers to develop complete processes for the convenient manufacturing of many products.
The developed system also leverages well-known advantages of continuous manufacturing technologies to reduce footprint and environmental impact, improve quality control, facilitate process scale-up and scale-out and reduce cycle time. Moreover, the fast response to changes in parameter settings characteristic of continuous manufacturing systems will accelerate product and process development, allowing for faster release of new products.
In this work, a novel continuous pharmaceutical manufacturing process for liquid dosages forms has been developed. The developed pilot-plant is situated at Rutgers University. The manufacturing process has been modelled to develop a digital twin for virtual experimentations. The work utilized advanced modeling approaches to enable a higher-level ability to understand injectable drug product manufacturing.
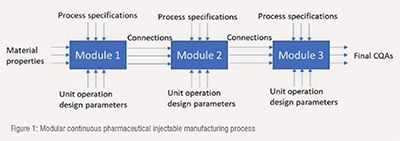
The continuous pharmaceutical injectable manufacturing process has three flexible modules as shown in Figure 1. In module 1, the API is conditioned in a preconditioning tank. This involves either dissolving powder API, or diluting an API liquid feedstock, to generate a solution with the desired concentration. Solutions of all remaining formulation ingredients are dispensed from additional refillable tanks. All ingredients are pumped at controlled mass flow rates to module 2. The module 2 consist of a static mixer, a homogenizer, an ultrafiltration, diversion system and a surge tank. The ultrafiltration membrane is used for purification and sterilization. Module 3 consist of vial filling, labelling, and caping system. The outlet stream from ultrafiltration unit is pumped into a reservoir tank (surge capacity), from where it is metered-fed into vial filling machine. The filled vials are then transferred to a capping station.
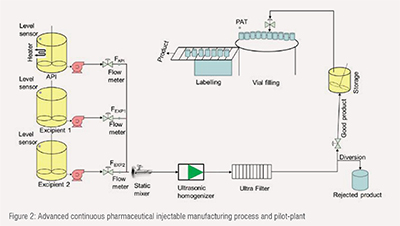
A continuous injectable manufacturing process has been developed as shown in Figure 2. The process takes the API synthesized and purified in a continuous modular API manufacturing process3 either in powder form or as a solution in the final liquid ingredient of the formulation and turns it into a finished liquid product. The existing commercially available API can be also used as a starting material for this process. The process is composed of three modules as described in following section.
3.1. Module 1: Feeding and dispensing system
In this module, the API is conditioned in a preconditioning tank. This involves either dissolving powder API, or diluting an API liquid feedstock, to generate a solution with the desired concentration. Solutions of all remaining formulation ingredients are dispensed from additional refillable tanks. All ingredients are pumped at controlled mass rates. Module 1 have sub-modules. Each submodule consists of an integrated re-filling system, feed tank, and pump. This submodule can be easily numbered up depending on the number of starting materials (APIs, Excipients) to be used in the formulation.
3.2. Module 2: Mixing and purification system
Module 2 consist of static mixer, ultrasonic homogenizer, ultrafiltration, real time diversion system, and surge tank. Static mixer is used to bring all the feeding streams from module 1 together and create a homogeneous solution via redial mixing. The main function of the ultrasonic homogenizer is to break down the agglomerates and larger particles and create a homogeneous solution of acceptable particle size distribution. The necessity of the homogenizer depends on the formulation and products, and it may or may not be needed. The module 2 has been designed in such a way so that homogenization process can be by-passed if needed. The next unit operation of module 2 is the ultrafiltration. This unit operation assures the destroying and removal of any surviving bacteria, viruses, and other impurities. There is a diversion system to divert the out of specification solution stream in real time. The diversion is based on inline measurement coupled with a predictive residence time distribution (RTD) model. The acceptable product stream from module 2 goes to a surge tank. The surge tank is used to make the balance in between module 2 and module 3.
3.3. Module 3: Vials filling, caping, and labelling system
The solution from surge tank is pumped to module 3. Module 3 uses a vial filling system to fill the vials. Each vial is automatically caped and labelled. The PAT is used to assure the critical quality attributes of each vial.
The central component of the 'digital twin' is the process flowsheet model, incorporating a mathematical representation of the three modules outlined in the preceding section. Additional modules can be seamlessly integrated as necessary. The primary module focuses on feeding and dispensing, encompassing models for a refill unit, feed tank, and pump.
The second module consist of the mathematical model of static mixer, ultrasonic homogenizer, ultrafiltration, diversion system, and surge tank. The last module consists of the model of vial filling system. Both digital twin model and computational fluid dynamics (CFD) models have been developed.
An advanced modular pharmaceutical manufacturing process and pilot plant has been developed for the continuous manufacturing of liquid dosages forms (injectables). The process is flexible and generic and can be adapted for a variety of injectable drug product manufacturing. A digital twin model library has been also developed that can be used for several applications including for scenario analysis, sensitivity analysis, dynamic optimization, and design of suitable control system.
This work is supported by the US Food and Drug Administration (FDA) under contract number 75F40122C00122.
References
1. Yu, L. (2016). Continuous Manufacturing Has a Strong Impact on Drug Quality. U.S. Food and Drug Administration (FDA). https://www.pharmaceuticalprocessingworld.com/continuous-manufacturing-has-a-strong-impact-on-drug-quality/
2. Singh, R., Sahay, A., Fernando Muzzio, Ierapetritou, M., Ramachandran, R. (2014). A systematic framework for onsite design and implementation of the control system in continuous tablet manufacturing process. Computers & Chemical Engineering Journal, 66, 186-200. http://dx.doi.org/10.1016/j.compchemeng.2014.02.029
3. Singh, R. (2023). Advanced continuous manufacturing of drug. Pharmaceutical Technology. Trends in Manufacturing, 20-26.